Found 3 results
Article
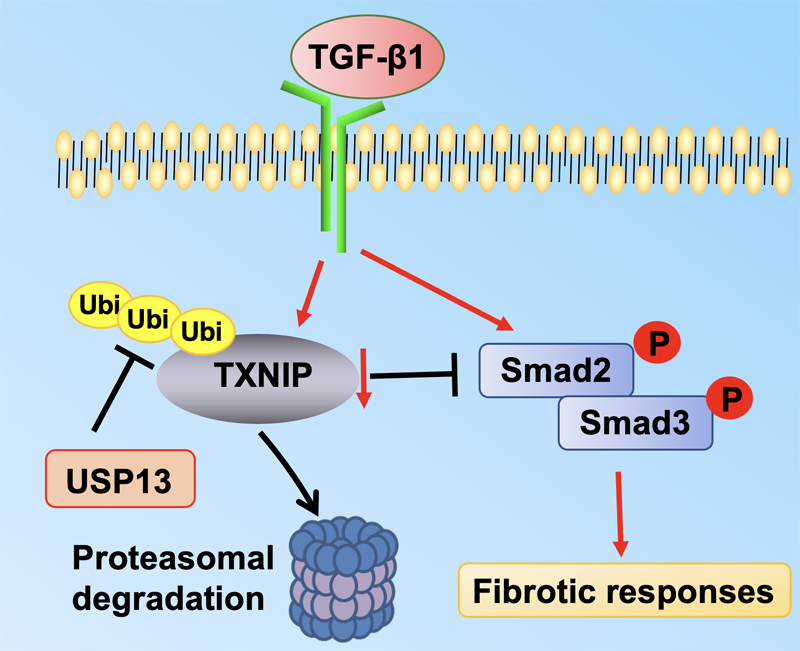
01 February 2024Molecular Regulation of Transforming Growth Factor-β1-induced Thioredoxin-interacting Protein Ubiquitination and Proteasomal Degradation in Lung Fibroblasts: Implication in Pulmonary Fibrosis
Thioredoxin-interacting protein (TXNIP) plays a critical role in regulation of cellular redox reactions and inflammatory responses by interacting with thioredoxin (TRX) or the inflammasome. The role of TXNIP in lung fibrosis and molecular regulation of its stability have not been well studied. Therefore, here we investigated the molecular regulation of TXNIP stability and its role in TGF-β1-mediated signaling in lung fibroblasts. TXNIP protein levels were significantly decreased in lung tissues from bleomycin-challenged mice. Overexpression of TXNIP attenuated transforming growth factor-β1 (TGF-β1)-induced phosphorylation of Smad2/3 and fibronectin expression in lung fibroblasts, suggesting that decrease in TXNIP may contribute to the pathogenesis of lung fibrosis. Further, we observed that TGF-β1 lowered TXNIP protein levels, while TXNIP mRNA levels were unaltered by TGF-β1 exposure. TGF-β1 induced TXNIP degradation via the ubiquitin-proteasome system. A serine residue mutant (TNXIP-S308A) was resistant to TGF-β1-induced degradation. Furthermore, downregulation of ubiquitin-specific protease-13 (USP13) promoted the TGF-β1-induced TXNIP ubiquitination and degradation. Mechanistic studies revealed that USP13 targeted and deubiquitinated TXNIP. The results of this study revealed that the decrease of TXNIP in lungs apparently contributes to the pathogenesis of pulmonary fibrosis and that USP13 can target TXNP for deubiquitination and regulate its stability.
Article
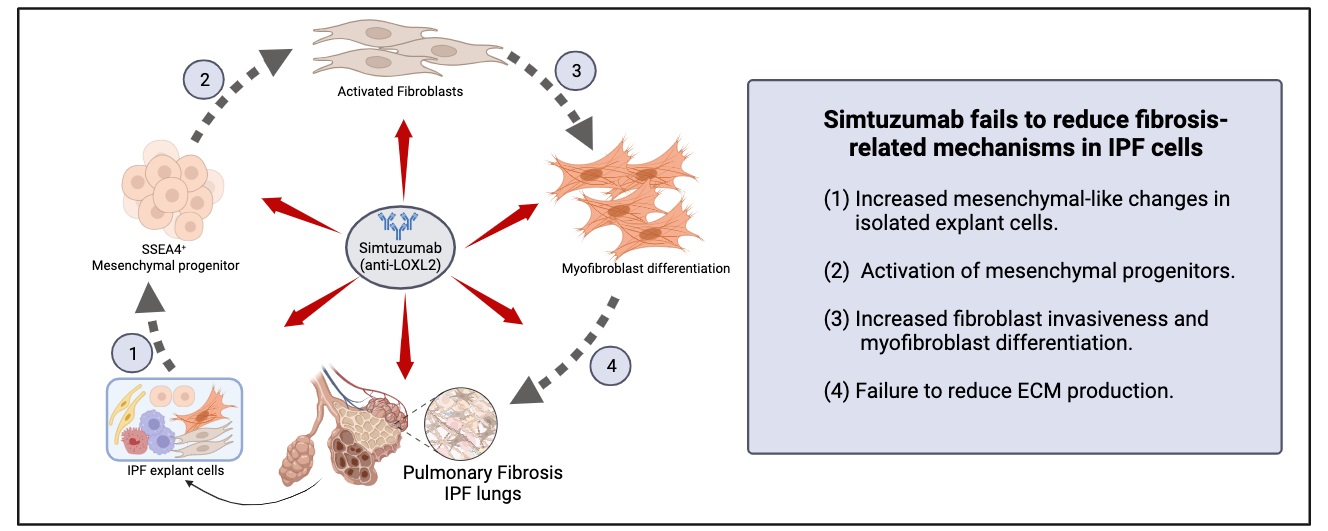
28 November 2023Translational Studies Reveal the Divergent Effects of Simtuzumab Targeting LOXL2 in Idiopathic Pulmonary Fibrosis
The composition of extracellular matrix (ECM) is altered during pathologic scarring in damaged organs including the lung. One major change in the ECM involves the cross-linking of collagen, which promotes fibroblast to myofibroblast differentiation. We examined the role of lysyl oxidase (LOX)-like 2 in lung progenitors and fibroblasts cultured from normal or IPF lung samples and in a humanized mouse model of IPF using a monoclonal antibody (Simtuzumab). Primary lung fibroblasts from normal donor lungs and IPF lung explants were examined for expression of LOXL2. Targeting LOXL2 with Simtuzumab on normal and IPF fibroblasts was examined both in vitro and in vivo for synthetic, functional, and profibrotic properties. LOXL2 was increased at transcript and protein level in IPF compared with normal lung samples. In a dose-dependent manner, Simtuzumab enhanced differentiation of fibroblasts into myofibroblasts. Inhibition of LOXL2 also enhanced fibroblast invasion and accelerated the outgrowth of fibroblasts from dissociated human lung cell preparations. Finally, preventative or delayed delivery of Simtuzumab enhanced lung fibrosis in a humanized mouse model of pulmonary fibrosis. Consistent with its failure in a Phase 2 clinical trial, Simtuzumab exhibited no therapeutic efficacy in translational in vitro and in vivo assays.
Article
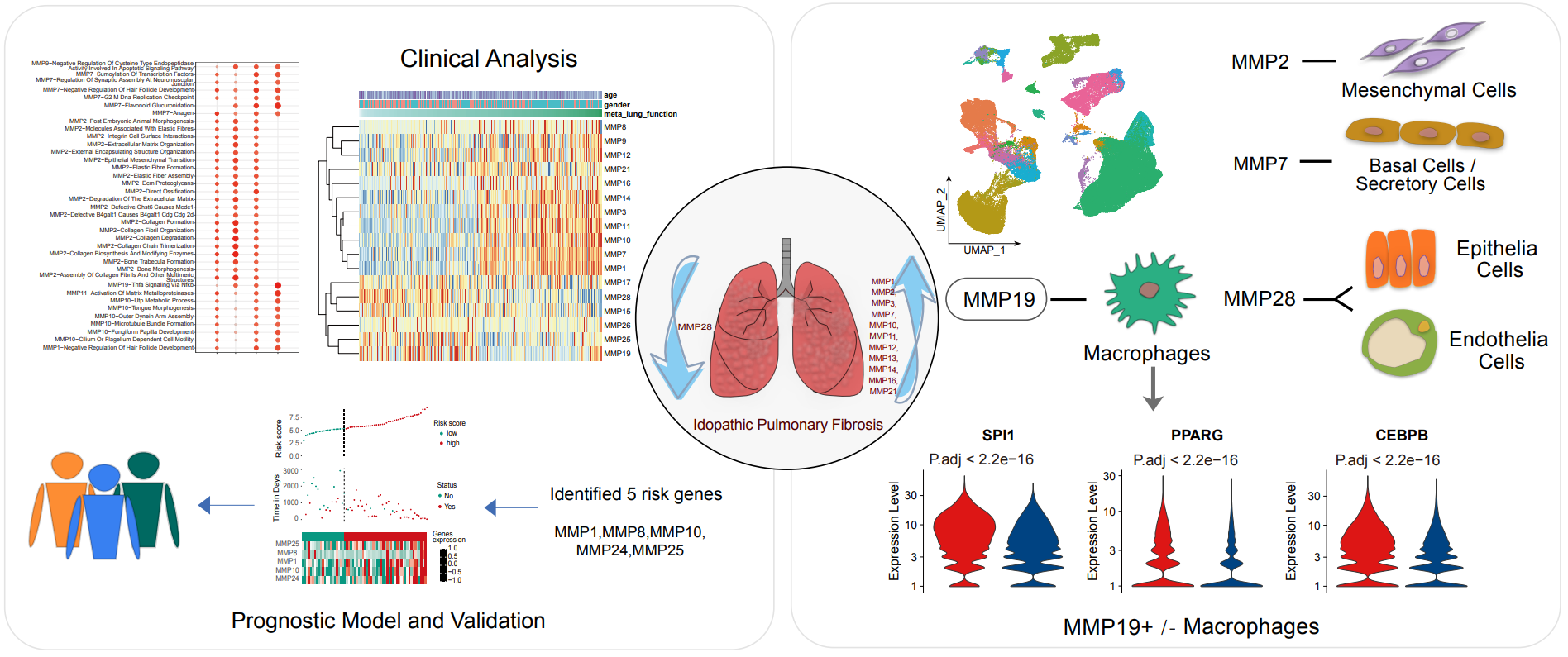
19 May 2023Comprehensive Landscape of Matrix Metalloproteinases in the Pathogenesis of Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a progressive, chronic interstitial lung disease with unknown etiology. Matrix metalloproteinases (MMPs) are involved in fibrotic lung tissues, contributing to the initiation, progression, or resolution of chronic inflammatory disease. In present study, comprehensive changes of MMPs expressions were investigated in IPF by integrative analysis of single-cell transcriptome and bulk transcriptome data. 24 of MMPs were altered and the changes could significantly distinguish IPF from normal subjects and other lung diseases. Among them, MMP1, MMP7 and MMP19 were closely associated to lung functions, susceptibility and alveolar surface density. MMP1 and MMP7 as potential diagnostic indicators, MMP1 and MMP19 as prognostic markers in IPF could accurately predict disease progression. Devolution of MMPs at single-cell resolution, MMP19 was highly expressed in macrophages and markedly interfered with TNF signaling pathway which synchronizes fibrotic microenvironment. MMP19+ macrophages were significantly different from MMP19- macrophages in energy metabolism and immune function. The interaction of MMP19+ macrophages with hyperplastic AT2 was mediated by TNFSF12-TNFRSF12A, and further activated the TNFRSF12A receptor to affect cell glucose metabolism and mitochondrial function. In summary, MMPs has great application potential in the diagnosis, treatment, and prognosis of IPF.